Miss Ferguson, 23 year old student presented with bloody diarrhoea. Her endoscopy showed
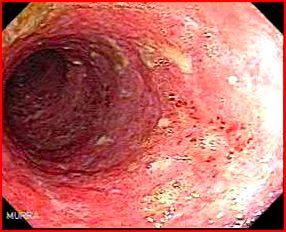
What is the diagnosis?
Moderately active colitis
The picture shows loss of vascular pattern, erythema, granularity and small ulcers
Discuss the endoscopic features of Crohn’s disease?
Crohn’s disease (CD) can affect any part of the GI tract. The commonest involvement is ileocaecal (approx 50%) and then small bowel involvement alone (30% approx). Colonic Crohn’s disease without small bowel involvement is less common (5–25%).
Endoscopic features include:
- Ulcers- aphthous ulcers is the most characteristic finding in CD. Aphthous ulcer is a small (max 5mm) superficial ulcer surrounded by a characteristic tiny rim of erythema. They may enlarge and give rise to larger and deeper ulcerations of various shapes: longitudinal, tortuous or serpiginous ulcerations.
- Cobble stoning- The mucosa lying between long linear ulcerations can be normal or very oedematous, reddish and hyperplastic, almost polyp like. This is referred to as the cobblestone appearance
- Patchiness- segmental affection with other areas completely spared (i.e. skip lesions)
- Transmural inflammation may cause strictures and fistulae
- Quiescent disease: it shows diminished or disturbed vascular pattern. In patients with more extensive disease healing may be more irregular and hypertrophic zones may alternate with areas of atrophy. This gives rise to the so called pseudopolyps.
Discuss the endoscopic features of UC?
The inflammation in UC extends typically from the anal verge up to a variable distance, which can change during the course of the disease. A large Scandinavian study showed that up to 40% of the patients had extension of their disease. Patients with initial extensive colitis showed regression over time in 44%.
Endoscopic appearances
- The inflammation is continuous and circumferential from the anal verge up.
- The mucosa shows
- Decreased or absent vascular pattern
- Oedema (causes shiny appearance)
- Erythema (reddening of the mucosa)
- Granularity (micro nodular mucosal surface)
- Friability (Bleeding mucosa either at contact or spontaneously)
In moderate UC, one can appreciate
- Erosions (Small superficial defect in the mucosa, of a white or yellow colour, with a flat edge) and micro ulcerations.
In more severe attacks shallow ulcerations may develop. Only rarely do deeper ulcerations and luminal narrowing occur.
- Signs of chronic inflammation and healing- pseudopolyps and an attenuated or loss of vascular pattern.
How do you differentiate UC and CD?
A complete colonoscopy generally allows a correct differentiation between Crohn’s disease, ulcerative colitis, and ‘indeterminate colitis’ in the majority.
The most distinctive endoscopic features in the differential diagnosis are discontinuous involvement, anal lesions, and cobble stoning of the mucosa for Crohn’s disease and erosions, micro ulcers, and granularity for ulcerative colitis.
Some patients show abnormalities that are suggestive of both conditions. Most of the patients have an UC-like endoscopy with one or more features possibly suggesting Crohn’s disease. These so-called ‘indeterminate features’ included anal abnormalities (such as skin tags, an unusual fissure or an abscess), rectal sparing, skip areas, and deeper ulcerations. In these patients, the term ‘indeterminate colitis’ should be used as a reminder that the differential diagnosis is not completely clear.
Discuss the endoscopic assessment of the severity of IBD?
The correlation between endoscopic severity and clinical activity is often poor in Crohn’s disease but NOT in ulcerative colitis.
UC: Several endoscopic scoring systems have been developed. Rutegard scoring system:
0- Normal
1- Non visible vessels, friability/
2- Ulcers, single or scattered
3- Confluent ulcers with pus
Endoscopic assessment of severity in CD is unhelpful as it correlates poorly to clinical activity.
Discuss endoscopic assessment post surgical resection in CD?
One of the most underutilized applications of endoscopy in CD is the assessment of the ileocolonic anastomosis following resection. The endoscopic appearance of the ileocolonic anastomosis 6 months following surgery can be used to risk-stratify patients, helping identify those who might benefit from an earlier escalation in therapy.
Classification of Endoscopic CD Recurrence Following Resection- Rutgeert’s anastomotic Score
| Rutgeert’s Anastomotic Score | Prognosis |
|---|---|
| 0- No inflammation | predict a low risk of recurrent symptomatic disease over the next few years |
| 1- Less than 5 aphthous ulcers | predict a low risk of recurrent symptomatic disease over the next few years |
| 2- Greater than 5 aphthous ulcers, with normal intervening mucosa -or- Skip lesions of larger lesions -or- Lesions confined to the anastomosis | Intermediate risk |
| 3- Diffuse Aphthous ileitis with diffusely inflamed ileal mucosa | Develops recurrent symptoms over the next several years. |
| 4- Diffuse inflammation with larger ulcers, nodularity, or Narrowing | Develops recurrent symptoms over the next several years. Almost a third of patients with a score of 4 required a second resection within 3 years |
Although sufficiently powered studies have not been performed, it is believed that early evaluation and appropriate treatment escalation can prevent symptomatic recurrence and the need for surgical intervention.
What is a DALM and what is its significance?
Dysplasia may occur within or near raised plaque-like lesions, nodules, polyps, or masses. This is referred to as dysplasia-associated lesion or mass [DALM]. Invasive carcinoma has been found on resection specimens in up to 60% of patients with DALM lesions.
UC patients also develop sporadic colonic adenomas.
You find a polyp/polypoid lesion in the patient. The question is, is it a DALM lesion or is just sporadic adenoma that confers no higher risk for adenocarcinoma? This situation is fairly common. A suggested strategy is:
- Polypectomy if feasible
- Biopsies from around the polypectomy site (if dysplasia- this is DALM)
- Extensive biopsies of the rest of the colon
Treatment
- Adenoma (regardless of whether they occurred in an area of the colon involved with colitis) with no dysplasia either around the polypectomy site or from the rest of the colon- routine polyp surveillance
- Adenoma with dysplasia around the polypectomy site or in other colonic biopsies- colectomy recommended.
- Unresectable polyp/polypoid mass or adjacent or remote dysplasia- colectomy is recommended. Non-adenoma-like polyps or other masses occurring in an inflammed area of colon, with or without surrounding dysplasia, should be considered as potentially containing invasive carcinoma
Discuss some of the confusing issues in endoscopic diagnosis of IBD?
- Certain errors are commonly made in the interpretation of endoscopic findings in patients with IBD. While most physicians recognize that rectal therapy with steroid or mesalamine products can give rise to rectal sparing, it is less commonly recognized that systemic therapy can produce the same result. In the treated patient with UC, inflammation often appears segmental, often with relative rectal sparing.
- A few patients with uncomplicated left sided UC will be found to have a small area of chronically inflamed mucosa surrounding the appendiceal orifice (cecal patch). Although technically skip lesions, these “cecal patches” do not predict other findings consistent with CD and are considered a typical finding in either disease.
- “Backwash ileitis”- A minority of UC patients with pancolitis sometimes have a short segment of mildly inflamed terminal ileum. This is typically not more than a few centimetres in length, and ulcerations are not usually seen. More severe inflammation, more extensive disease, or ileal inflammation in the absence of pancolitis provides evidence for the diagnosis of CD.
- The presence of “focally enhanced gastritis” is another source of confusion. While this was originally described in CD, it has been seen in patients with UC as well, and thus is little help differentiating the 2 diagnoses. Diffuse duodenitis in UC has also been reported, particularly in younger patients. While a formal diagnosis of UC or CD can be debated in these patients, they often have a benign course following colectomy with ileal pouch anal anastomosis (IPAA) formation.
Discuss the endoscopic features of pouchitis?
Although the inflammation remains limited to the pouch reservoir in the majority of patients, extension into the pre pouch ileum can occur.
The endoscopic features of pouchitis resemble those of UC: in an early stage erythema, fading of the vascular pattern, granularity, and friability appear. Later, punctiform mucosal haemorrhages and superficial ulcerations develop. Less frequently large isolated ulcers with normal surrounding mucosa can be found like in Crohn’s disease. Undiagnosed Crohn’s disease is a rare but important differential diagnosis particularly if large ulcers or fistulas are present.
Pouchitis generally involves most of the pouch reservoir and should be differentiated from ‘cuffitis’. This entity represents a recurrence of ulcerative colitis in the short cuff of the rectoanal transitional zone that has been preserved in case of a double-stapled pouch anastomosis. It usually extends over a distance of 1 cm. The endoscopic feature is that of a distal ulcerative proctitis with a clear demarcation to ileal pouch mucosa.
Discuss the histology in IBD?
UC- intense infiltration of the mucosa and submucosa with neutrophils and crypt abscesses, lamina propria with lymphoid aggregates, plasma cells, mast cells and eosinophils, and shortening and branching of the crypts. These features are not unique to ulcerative colitis. Except for crypt distortion, the cellular response can be in acute infectious colitis or CD.
Noncaseating granulomas, the hallmark of CD, are found in approx 30% of biopsy specimens and in approx 50% of surgical specimens. Of note, in order to provide convincing evidence of CD, granulomas must be separate from the crypts, as occasional mucin granulomas can be seen in either disease.
Ref






